Advance with Macrocyclics
Comprehensive Support for Your Program

Macrocyclics is focused on pre-clinical conjugation projects primarily with nuclear medicine applications. Our expertise lies in optimizing reaction conditions and and physicochemical analysis with every project plan customized to meet your program’s specific challenges and needs.
- Evaluate different conjugation chemistries to achieve desired construct
- Proof of Concept scale to multi-gram manufacturing
- Stability protocols to evauluate storage conditions and formulations
Conjugation is Chemistry!
Conjugation mechanisms are driven by fundamental reaction principles. Buffer selection is critical to achieve appropriate pH for conjugation while avoiding precipitation of the protein. Overall, the best conjugation mechanism is determined
by the ability to engineer the required conjugation motif onto the protein.

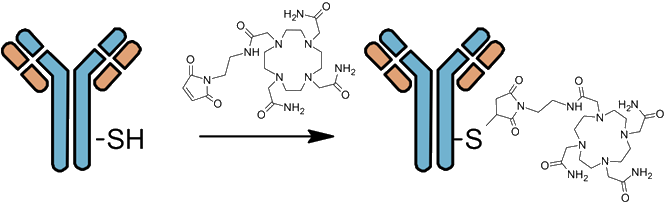
Site-specific mechanisms result in homogenous, defined constructs.
- ► Maleimide functional groups react with Cysteine
- ► Limited to CAR of 2 or 4 to retain stability
- ► Enzyme mediated and “click” reaction alternatives
Random conjugation targets lysine residues present on the primary amino-acid sequence.
- ► [-NCS] , [-NHS], and [[-COOH] functional groups
- ► Mechanism controlled by pH, temp., and hold time
- ► Results in a distribution of protein-conjugate ratios
Isothiocyanate Linkers [-NCS]
- Buffer Selection: Avoid Tris buffered saline (TBS) and ammonia solutions. The buffers will quench the isothiocyanate linker as they provide reactive amines. Instead, use Na2CO3 buffers to achieve pH > 8. Conjugation will proceed as low as pH 6.
- Stabilizers: Check your solutions for free arginine, often used to prevent protein aggregation, as the N-terminus will react and quench the isothiocyanate.
Maleimide Linkers [-mal]
- Avoid Aqueous Conditions: The maleimide linker will start to hydrolyze at pH > 5, and proceeds quickly under basic conditions.
Metal Contamination
- Metal-free reagents: Trace Na⁺, Ca²⁺, and Fe³⁺ can outcompete your radiometal during labeling. Use caution if added Water for Injection (WFI) unless it is verified to be metal free. Only use plastic and glass utensils as metals like Fe and Ni can be leeched from stainless steel.
Final SEC or Ultrafiltration
- Excess Residual Chelate: A failure to remove unconjugated chelating agent will appear as poor radiolabeling efficiency. Due to the extremely low molar amounts of radiometal used, complete removal of unconjugated chelator is critical.
Submit a request using our Contact From. Please provide a few key details to help us assess your chelator-antibody conjugation project such as:
🔬 Project Stage and Timeline: Timelines are critical. Please let us know when you need the final material and what the intended application is. The scope, lead times, and development strategy for clinical-phase research differ significantly from those for preclinical studies.
🧪 Chelator and Isotope Selection: Indicate which chelating agent and/or radioisotope you plan to use. If you're unsure, we’re happy to recommend suitable options based on your project goals and strategy.
🧬 Final Packaging Requirements: We can aliquot material into various vial types and sizes based on your preferences. For GMP clinical-stage programs, we also offer final fill and finish services using ISO-5 isolators.

